What Is The PH Level Of Windex - A Closer Look
Have you ever stopped to think about what makes your favorite window cleaner work its magic? It's pretty interesting, actually, how everyday liquids, including those we use for tidying up our homes, have a particular chemical characteristic that helps them get the job done. This characteristic is often described by something called pH, a simple measure that tells us if a liquid is more on the sour side, the slippery side, or somewhere right in the middle. Knowing a little about this can help us understand why certain cleaners are good for some tasks and not others, you know?
We often grab a bottle of something to make our glass sparkle without giving much thought to the science behind it. Yet, every single liquid we encounter, from the water we drink to the solutions we use for keeping things neat, possesses a specific pH. This numerical value, running from zero to fourteen, provides a quick way to gauge how a liquid might interact with different surfaces or even other substances. It's a fundamental concept that helps chemists, and really, anyone curious about how things work, make sense of the world around them, so.
When we talk about something like "what is the pH level of Windex," we're really asking about its chemical identity in a way. This little number, the pH, helps us classify liquids into broad groups: those that are on the acidic end, those that are basic or alkaline, and those that are considered neutral. Each of these categories behaves quite differently, and understanding these differences can actually be quite helpful for keeping our homes clean and safe. It's a pretty basic idea, but it has some really widespread implications, too it's almost.
Table of Contents
- Understanding pH - What is the pH Level of Windex?
- How Do We Measure What is the pH Level of Windex?
- Why Does the pH Level of Windex Matter?
- The pH Scale - Exploring What is the pH Level of Windex
- Strong Versus Weak - What is the pH Level of Windex?
- Everyday Examples - Beyond What is the pH Level of Windex
- pH and Your Body - What is the pH Level of Windex?
- The History Behind What is the pH Level of Windex
Understanding pH - What is the pH Level of Windex?
So, what exactly is pH? At its heart, pH is a way to quantify how many hydrogen ions are floating around in a liquid. The more hydrogen ions, the more acidic the liquid tends to be. Fewer hydrogen ions mean it leans towards the basic or alkaline side. It's a numerical way to express how sour or how slippery a liquid might feel or act. This concept, you know, gives us a way to talk about the chemical character of water-based liquids in a very clear manner. The full phrase for pH is "potential of hydrogen," which really just points to the concentration of these tiny hydrogen parts in a liquid, as a matter of fact.
The calculation behind pH is a bit mathematical, involving something called a logarithm. In simple terms, it's a negative log of the hydrogen ion concentration. This means that a small change in the pH number actually represents a very big change in the actual concentration of those hydrogen ions. For instance, a liquid with a pH of 6 has ten times more hydrogen ions than a liquid with a pH of 7, which is pretty significant. This logarithmic nature means that even a tiny shift on the pH scale can make a world of difference in how a substance behaves, really.
This definition of pH, which involves the negative logarithm of hydrogen ion concentration, typically applies best to solutions that are not too concentrated, often those with a strength of one mole per liter or less. For example, if you wanted to make liquids with different acidic qualities, you could simply use various strengths of something like hydrochloric acid. Each different strength would give you a different pH reading on the acidic part of the scale, sort of. It's a fundamental concept that helps us understand the chemical properties of many liquids we come across.
How Do We Measure What is the pH Level of Windex?
When someone asks about the pH of a particular cleaning agent, like "what is the pH level of Windex," they're asking for a specific number. To find this number, scientists and even home enthusiasts often use special tools. One common way is with a pH meter, which is a device that has a probe you dip into the liquid. This probe senses the concentration of hydrogen ions and then displays the pH value on a screen. It's a very straightforward way to get a precise reading, you know, much more so than guessing. These devices are quite useful in laboratories, but simpler versions are available for everyday use, too.
Another way to get a general idea of a liquid's pH is by using pH paper or litmus paper. These are strips of paper that have been treated with chemicals that change color depending on the pH of the liquid they touch. You dip the paper into the liquid, and then compare the color it turns to a color chart provided with the paper. This method isn't as precise as a meter, but it gives you a quick visual indication of whether something is acidic, neutral, or basic. It's a handy tool for quick checks, for instance, when you just need a general sense of the liquid's character.
These methods are what allow people in various fields, from chemistry to farming, to keep track of the pH of different liquids. Knowing how to measure pH is a pretty important skill for anyone working with solutions where acidity or basicity plays a role. It ensures consistency and helps predict how different substances will react when mixed. So, whether it's for something like understanding what is the pH level of Windex or testing the water in a swimming pool, these measurement tools are quite useful, actually.
Why Does the pH Level of Windex Matter?
The pH of a cleaning product, or any liquid for that matter, truly matters for several reasons. First off, it affects how well the product cleans. Some types of dirt and grime respond better to acidic cleaners, while others are more easily removed by basic ones. For example, soap scum, which is often a basic substance, is usually tackled more effectively by an acidic cleaner. On the other hand, greasy messes might break down better with a basic solution. So, the pH is pretty important for the product's effectiveness, you see.
Beyond cleaning power, the pH also plays a big role in safety. Liquids that are extremely acidic or extremely basic can be corrosive. This means they can cause harm to skin, eyes, or even the surfaces they are used on. Imagine using a very strong acid on a delicate countertop; it could cause damage. Similarly, a very strong basic cleaner might not be suitable for all materials. So, understanding the pH helps us use products safely and appropriately, protecting both ourselves and our belongings, really.
Moreover, the pH can influence how a product interacts with other chemicals. Mixing certain cleaners with different pH levels can sometimes create harmful fumes or reduce their effectiveness. This is why it's often advised not to mix different cleaning agents. Knowing the pH characteristics of the products you use, even if it's just a general idea of "what is the pH level of Windex" compared to other cleaners, can help prevent unintended chemical reactions and ensure a safer cleaning experience. It's a bit like knowing which ingredients go well together in cooking, but for chemicals, too it's almost.
The pH Scale - Exploring What is the pH Level of Windex
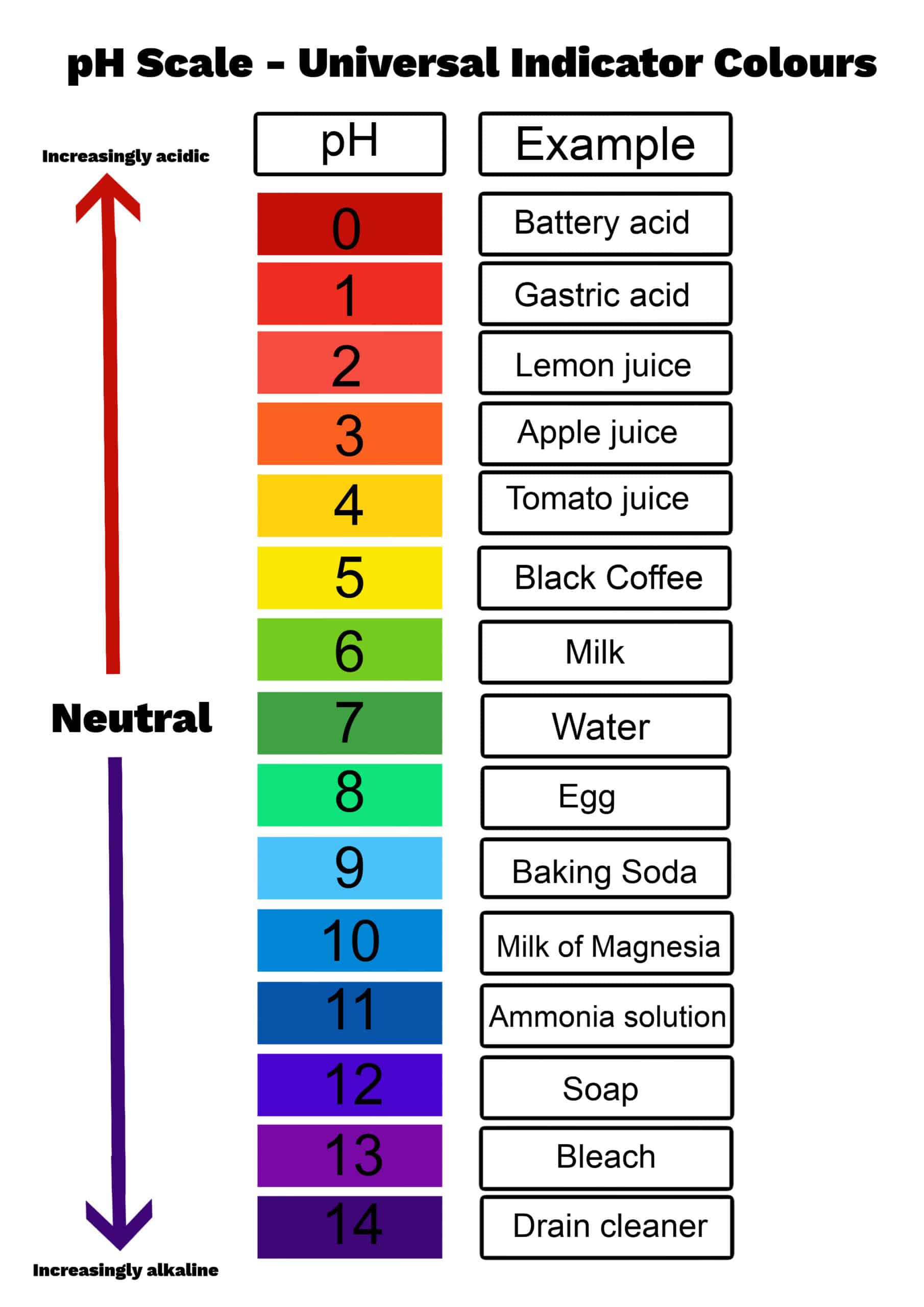
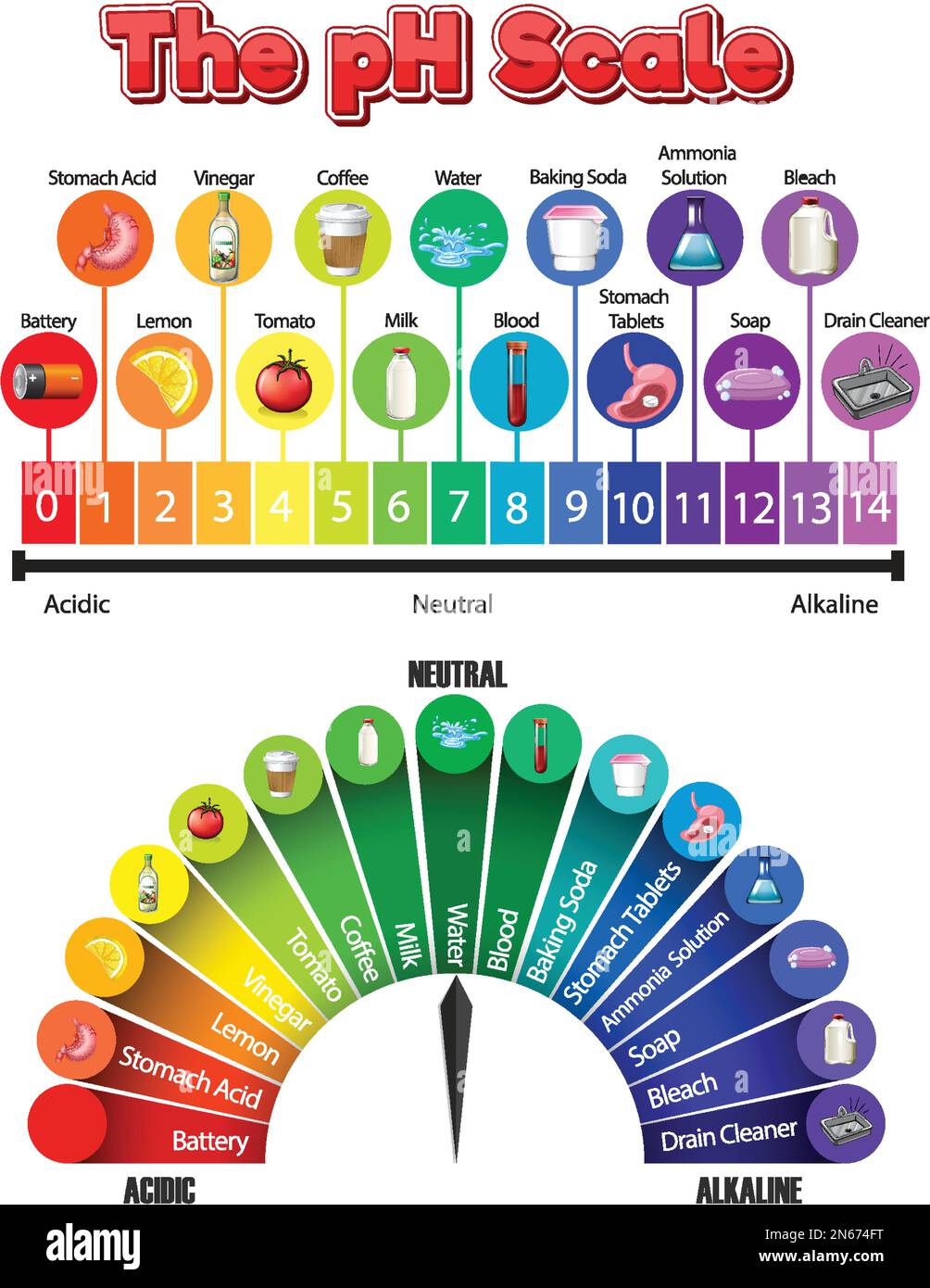
The pH scale is a numbered range that goes from 0 to 14. This scale helps us classify liquids based on their acidity or basicity. A pH of 7 is considered perfectly neutral. Pure water, for instance, typically has a pH of 7. Liquids with a pH below 7 are considered acidic, and the lower the number, the stronger the acid. For example, lemon juice or vinegar would fall into this acidic category, having numbers like 2 or 3. On the other hand, liquids with a pH above 7 are considered basic, or alkaline, and the higher the number, the stronger the base. Things like baking soda dissolved in water or household ammonia would be on this basic end of the scale, often around 9 or 10, in a way.
Thinking about this scale helps us place different substances. For instance, if we were to consider "what is the pH level of Windex," we'd be looking for where it sits on this 0-14 range. Most glass cleaners tend to be on the basic side, as alkalinity helps break down grease and grime often found on windows. However, they are usually not extremely basic, as that could damage surfaces or be unsafe for general use. They need to be effective but also relatively gentle. So, a cleaner like Windex would likely be somewhat basic, but not at the very top end of the scale, you know.
The scale itself is a clever way to show the vast differences in hydrogen ion concentration. Because it's a logarithmic scale, each whole number step represents a tenfold change in acidity or basicity. This means that a liquid with a pH of 10 is ten times more basic than a liquid with a pH of 9, and a hundred times more basic than a liquid with a pH of 8. This big jump with each number is why even small differences in pH can have noticeable effects on how a liquid behaves and what it's good for. It's a pretty powerful way to organize chemical information, basically.
Strong Versus Weak - What is the pH Level of Windex?
When we talk about acids and bases, we also consider if they are "strong" or "weak." A strong acid, like nitric acid, gives up almost all its hydrogen ions when put into water, making the solution very acidic very quickly. Strong acids, you see, cause a dramatic change in the pH. Similarly, strong bases, when added to water, produce a lot of hydroxide ions, which makes the solution very basic. The text mentions that diluting a strong base by a factor of ten to the power of 'n' will decrease its pH, but the diluted pH will still be somewhat close to the original, just a little lower, you know.
On the other hand, weak acids and weak bases don't fully break apart in water. They only release some of their hydrogen or hydroxide ions. This means they don't change the pH as drastically as strong ones do. For example, sulfuric acid, while strong in its first step of breaking apart, is not as strong in its second step. This characteristic of being "strong" or "weak" is pretty important for how chemicals are used. Cleaners, for instance, are often formulated with weak or moderately strong acids or bases to be effective without being overly aggressive or dangerous, as a matter of fact.
Understanding the difference between strong and weak is key when considering any chemical, including something like "what is the pH level of Windex." While Windex is effective, it's not meant to be extremely corrosive. This suggests it likely contains a moderately strong or weak base, rather than a very strong one. This balance allows it to clean glass surfaces without causing damage or posing an extreme risk to the user. It's a thoughtful approach to chemical formulation, ensuring both power and a degree of safety, anyway.
Everyday Examples - Beyond What is the pH Level of Windex
pH isn't just a concept for chemistry labs or for figuring out "what is the pH level of Windex." It's something that touches our lives every single day, in countless ways. Think about the food we eat, for instance. Lemon juice is quite acidic, which is why it tastes sour and is great for tenderizing meats or adding a zing to dishes. Baking soda, on the other hand, is basic and is often used in baking to help things rise or to neutralize acidic flavors. Our bodies also have a very specific pH balance that needs to be maintained for good health. So, it's pretty much everywhere, literally.
In agriculture, the pH of the soil is incredibly important for growing plants. Different plants prefer different soil pH levels. Some thrive in slightly acidic soil, while others need more alkaline conditions. Farmers and gardeners regularly test their soil's pH to make sure it's just right for what they want to grow. If the pH is off, the plants might not be able to absorb the nutrients they need, even if those nutrients are present in the soil. This shows how crucial pH is for life itself, you know.
Even in medicine, pH plays a vital role. Many bodily fluids, like blood, have a very narrow pH range that they need to stay within for our bodies to function properly. Our blood, for instance, typically has a pH between 7.2 and 7.4, which is very close to neutral. If this balance gets too far off, it can lead to serious health issues. Medicines are also often formulated to be effective at specific pH levels, and their stability can depend on it. So, whether it's understanding a cleaning product or how our bodies work, pH is a truly fundamental concept, as a matter of fact.
pH and Your Body - What is the pH Level of Windex?
Our bodies are pretty amazing at keeping their internal environment stable, and pH is a big part of that. The pH of our blood, as mentioned, is usually kept in a very tight range, around 7.2 to 7.4. This is a slightly basic range, and it's essential for all the chemical reactions that happen inside us. If our blood pH gets too acidic or too basic, it can really mess with how our cells and organs work. What we consume, both food and drink, can have an impact on our body's pH, though our bodies have strong systems to keep it balanced, basically.
Proteins in our bodies, which do a lot of the work, are also very sensitive to pH. The text mentions that if the liquid around a protein has a pH lower than the protein's "isoelectric point" (PI), the protein will gain positive charges. If the pH is higher than its PI, it will lose positive charges and become negatively charged. At a specific pH, where the tendency to gain or lose charges is equal, proteins can be quite stable. This sensitivity means that even small changes in pH can affect how proteins fold and function, which is critical for health. So, it's a pretty delicate balance, you know.
Beyond blood, other body fluids and even the solutions used in medical settings are carefully monitored for pH. For example, solutions used to wash cells before medical procedures or for transporting tissues often have a pH similar to that of the human body, around 7.2-7.4. They are also made to have an osmotic pressure similar to blood and are free of certain ions like calcium and magnesium, to be gentle on cells. This careful control of pH in biological and medical contexts highlights just how important this simple number is for life itself, really.
The History Behind What is the pH Level of Windex
The concept of pH as we know it today wasn't always around. It was introduced by a Danish chemist named Søren Sørensen back in 1909. He needed a simple way to express the acidity of solutions when he was working on brewing beer, and he came up with this clever scale. The 'p' in pH actually stands for the German word "potenz," which means "power" or "concentration." So, it literally means the "power of hydrogen" or "concentration of hydrogen," which is quite fitting, you know.
Before Sørensen, scientists could measure acidity, but there wasn't a universal, easy-to-understand scale that everyone could use. His invention made it much simpler to compare the acidic or basic nature of different liquids. This standardization was a pretty big deal for chemistry, biology, and even agriculture, as the text points out. It allowed researchers and practitioners across various fields to communicate about the chemical properties of solutions in a clear and consistent manner, which was a real step forward, so.
The adoption of the pH scale truly helped advance scientific understanding and practical applications. From figuring out the best conditions for chemical reactions to ensuring the right environment for cell growth in a lab, pH became a fundamental measurement. So, when we discuss something like "what is the pH level of Windex" today, we are using a system that has a rich history and has been incredibly useful in shaping our understanding of the chemical world around us. It's a testament to how a simple idea can have a truly widespread impact, honestly.
What is the pH Scale?

Premium Vector | Chart ph acidic, neutral and alkaline scale. Ph value
The pH scale of common chemicals illustration Stock Vector Image & Art